Education and Training
Petros Christopoulos is Professor of Medicine at Heidelberg University and Head of Scientific Coordination for the Thoracic Oncology Program in the Thoraxklinik at Heidelberg University Hospital. He has served as Principal Investigator in several clinical and translational research studies and is responsible for the weekly thoracic molecular tumor board and the subsequent treatment of patients with thoracic malignancies using novel compounds off-label or within expanded access programs. After studying medicine in the University of Athens, Greece, he completed training in Internal Medicine, Emergency Medicine, Hematology, Hematopoietic Cell Transplantation, Medical Oncology, Genetic Counseling, and Palliative Care at the University Hospitals of Freiburg im Breisgau and Würzburg in Germany. As a fellow, he participated in several phase 1, 2, and 3 clinical trials and applied state-of-the-art methods of cellular and molecular immunology to characterize the T-cell status of patients with various malignancies.
Expertise
A major focus of his research lies in the systematic integration of clinical with genetic, pathologic, immunologic, and radiologic data to refine patient stratification, improve disease monitoring and identify novel therapeutic targets, which are subsequently translated into advanced preclinical models and investigator-initiated phase 2 clinical trials. The power of this approach was demonstrated in a pivotal work connecting the National Center for Tumor diseases (NCT), Institute of Pathology Heidelberg and German Cancer Research Center (DKFZ), which defined molecular risk in ALK-rearranged non-small-cell lung cancer (NSCLC) utilizing deeply annotated patient cohorts alongside genetically modified mice, and now employs state-of-the-art longitudinal profiling to improve patient management within the prospective multicenter ABP study (NCT04318938). Similar strategies are currently applied to dissect other entities as well, for example analyze lung cancers harboring EGFR exon 20 insertions or receiving immunotherapy. A second main field is the characterization of systemic immune dysregulation associated with thoracic malignancies. Multicolor flow cytometry, gene expression and TCR-profiling, proteomic and T-cell functional assays are being established and applied in combination to improve understanding of tumor immunology, immunotherapeutic efficacy, and the development of immune-related toxicities, while an important subproject explores thymoma-related immunodeficiency.
- Molecular stratification in Thoracic Oncology
- Characterization of the systemic immune dysregulation associated with thoracic malignancies
- Thymoma-related immunodeficiency
Lung Cancer
- The impact of TP53 co-mutations and immunologic microenvironment on outcome of lung cancer with EGFR exon 20 insertions. Christopoulos P, Kluck K, Kirchner M, Lüders H, Roeper J, Falkenstern-Ge R, Szewczyk M, Sticht F, Saalfeld F, Wesseler C, Hackanson B, Dintner S, Faehling M, Kuon J, Janning M, Kauffmann D, Kazdal D, Kurz S, Eichhorn F, Bozorgmehr F, Shah R, Tufman A, Wermke M, Loges S, Brückl W, Schulz C, Misch D, Frost N, Kollemeier J, Reck M, Griesinger F, Grohe C, Liern-Hong J, Lin M, Budczies J, Stenzinger A, Thomas M. Eur J Cancer 2022 May 19;170:106-118. doi: 10.1016/j.ejca.2022.04.020.
- Longitudinal therapy monitoring of ALK-positive lung cancer by combined copy number and targeted mutation profiling of cell-free DNA. Dietz S,* Christopoulos P,* Yuan Z, Angeles AK, Gu L, Volckmar AL, Ogrodnik S, Janke F, Fratte CD, Zemojtel T, Schneider MA, Kazdal D, Endris V, Meister M, Muley T, Cecchin E, Reck M, Schlesner M, Thomas M, Stenzinger A, Sültmann S. EBioMedicine 2020 Dec;62:103103. doi: 10.1016/j.ebiom.2020.103103.
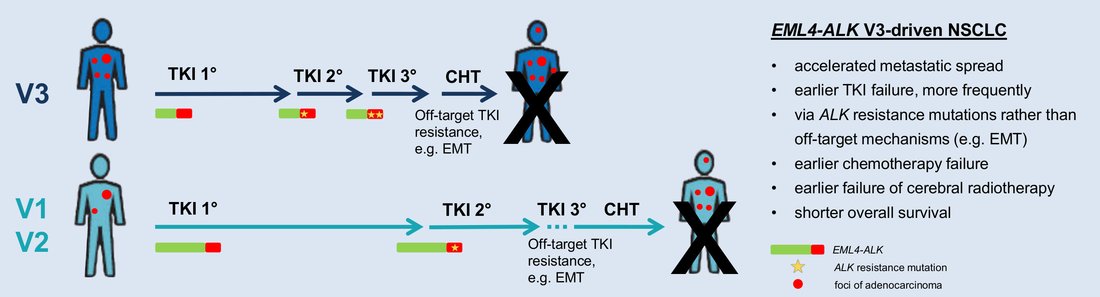
- Identification of a highly lethal V3+TP53+ subset in ALK+ lung adenocarcinoma. Christopoulos P, Kirchner M, Bozorgmehr F, Endris V, Elsayed M, Budczies J, Ristau J, Penzel R, Herth FJ, Heussel CP, Eichhorn M, Muley T, Meister M, Fischer J, Rieken S, Lasitschka F, Bischoff H, Sotillo R, Schirmacher P, Thomas M, Stenzinger A. Int J Cancer 2019 Jan 1;144(1):190-199. doi: 10.1002/ijc.31893 .
- Large cell neuroendocrine lung carcinoma induces peripheral T-cell repertoire alterations with predictive and prognostic significance. Christopoulos P, Schneider MA, Bozorgmehr F, Kuon J, Engel-Riedel W, Kollmeier J, Baum V, Muley T, Schnabel PA, Bischoff H, Grohé C, Serke M, Thomas M, Fisch P, Meister M. Lung Cancer 2018 May;119:48-55. doi: 10.1016/j.lungcan.2018.03.00.
- A novel thymoma-associated immunodeficiency with increased naive T cells and reduced CD247 expression. Christopoulos P, Dopfer EP, Malkovsky M, Esser PR, Schaefer HE, Marx A, Kock S, Rupp N, Lorenz MR, Schwarz K, Harder J, Martin SF, Werner M, Bogdan C, Schamel WA, Fisch P. J Immunol. 2015 Apr 1;194(7):3045-53. doi: 10.4049/jimmunol.1402805 .
- Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Christopoulos P, Pfeifer D, Bartholomé K, Follo M, Timmer J, Fisch P, Veelken H. Blood 2011 Apr 7;117(14):3836-46. doi: 10.1182/blood-2010-07-299321
Lena Gaissmaier | Doctoral student | ||
Mariam Elshiaty | Doctoral student | ||
Hannah Schindler | Doctoral student | ||
Fabienne Lusky | Doctoral student | ||
Tamara Hedinger | Technical Assistant | ||
Silke Winter | Technical Assistant |
Lung Research - Projects
- Defining molecular risk in ALK+ and other subsets of non-small-cell lung cancer.
After establishment of the ALK fusion variant and TP53 comutations as the first molecular risk factors in ALK+ NSCLC in close cooperation with the Institute of Pathology and the DKFZ, the group is now dissecting the role of other properties, such as gene expression and epigenetic tumor profiles and the immunologic microenvironment for the development of therapy resistance and patient survival. Furthermore, the EGFR+ and immunotherapy-treated NSCLC are analyzed in a similar manner. Subsequently, the results are utilized for the development of better methods for molecular monitoring and for improvement of the preclinical models in cooperation with the DKFZ. - Characterization of the systemic immune dysregulation in thoracic malignancies
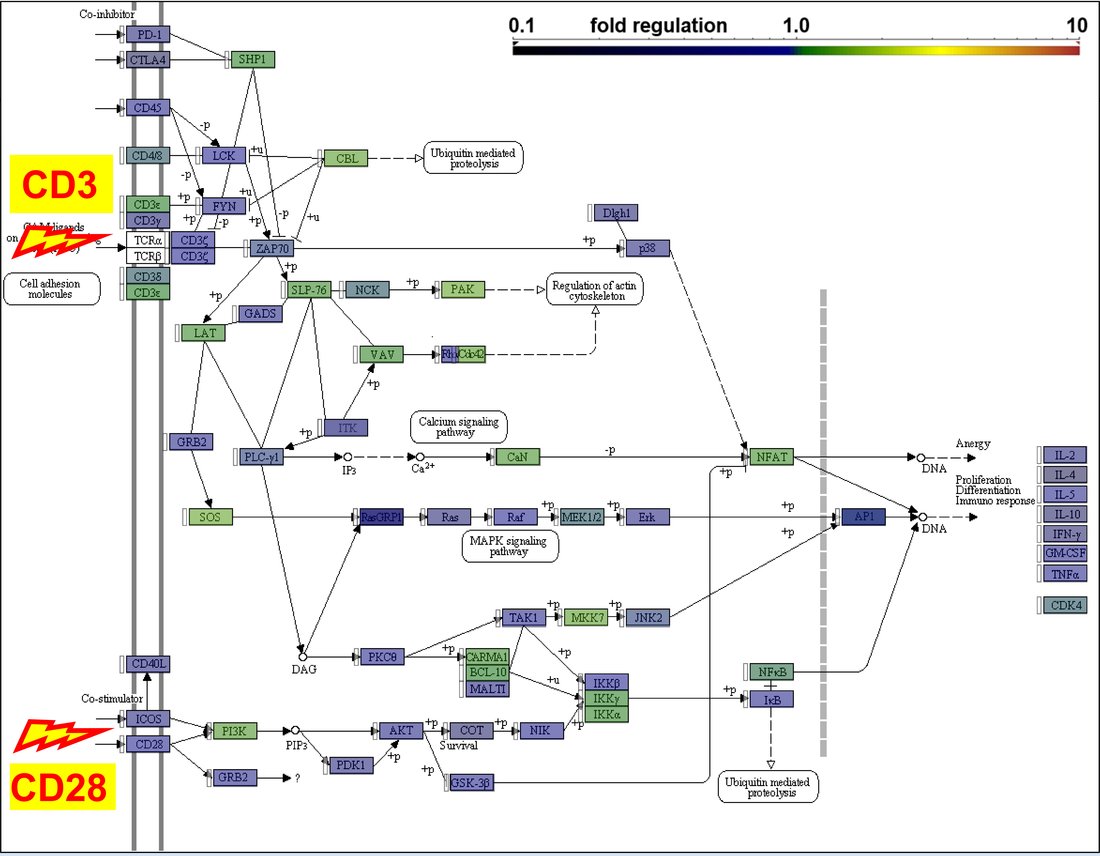
Patient samples are collected longitudinally and analyzed with multiple methods (multicolor flow cytometry, gene expression- and TCR-profiling, proteomic and T-cell functional assays) in order to identify and characterize subclinical immune defects in patients with NSCLC and other thoracic malignancies. These are of key importance for guidance of immunotherapy and the development of novel treatment strategies. - Thymoma-associated immunodeficiency
The immunodeficiency of cortical thymomas due to reduced CD247 expression that was identified during previous projects is explored further at the molecular level and analyzed in conjunction with other clinical and immunologic parameters.